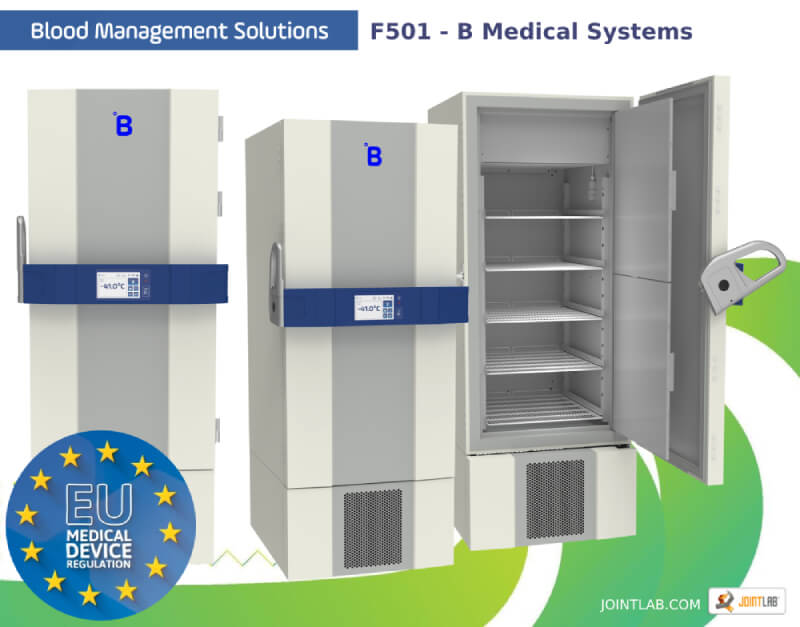
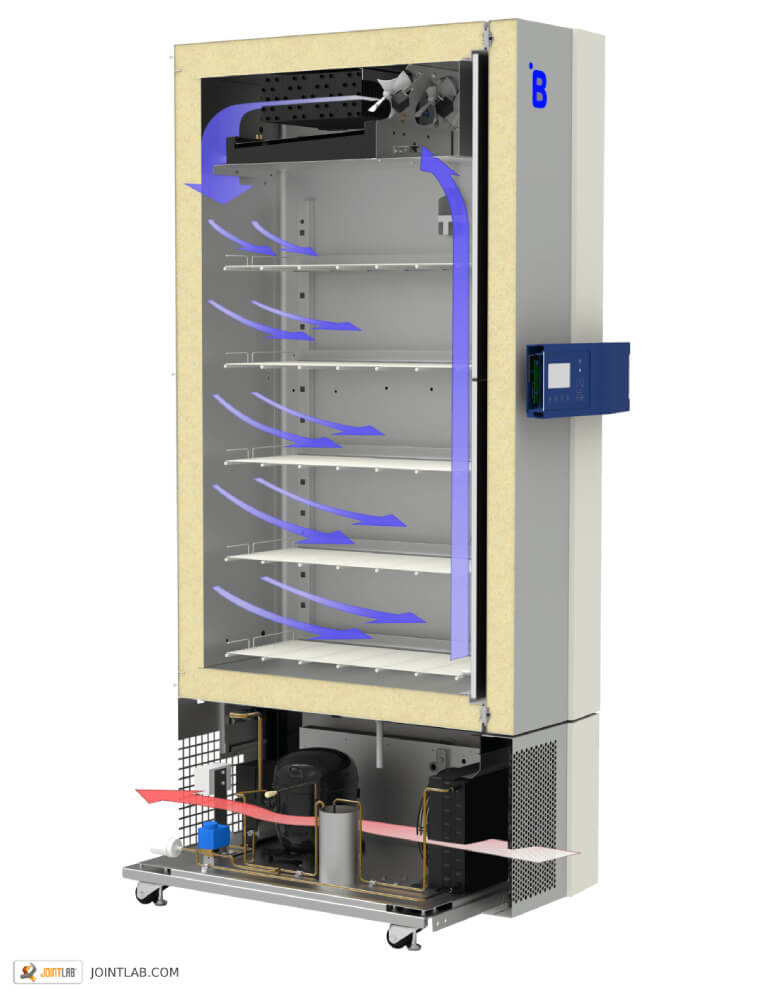
Plasma freezer F501 by B-Medical-Systems
991.8510.03
Delivery in 12/15 days
€8,964.00
VAT excluded
Price including VAT: €10,936.08
Taxes calculated 22%. Pls. keep in mind, taxes vary by country: France taxes will be 20%, in Spain 21% etc., out EC 0% and if you are a company always 0%
634 liter plasma freezer with adjustable temperature from -32°C to -41°C for safe storage of blood plasma or blood components at temperatures below -27°C.
- Temperature °C
- -41...-32
- External dimensions mm L x P x A
- 845 x 1039 x 1988
- Internal dimensions mm L x P x A
- 593 x 738 x 1173
- Control panel
- Color display touchscreen
- Internal capacity
- 634 / 514 liters Gross / Net
- Shelves and drawers
- 5 shelves
- Power W
- 1300W
- Climate class
- SN-T from +10°C to +43°C
- Net internal capacity
- 514
- Absorption
- 6.6 (kWh/24h)
- Weight kg
- 301
- Supply
- 220-240V / 50 Hz
Options or connected products